Ozempic Linked to Severe Health Conditions
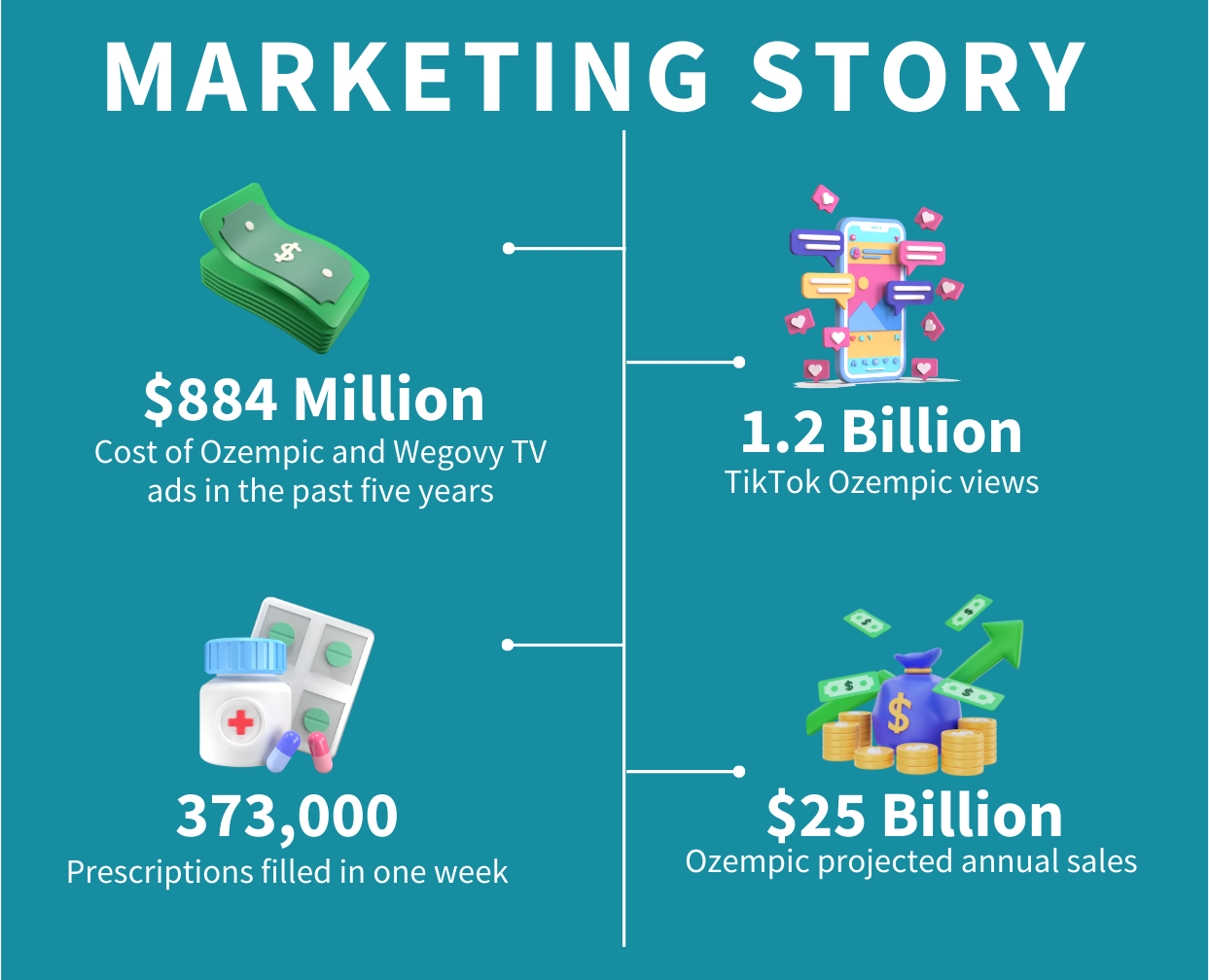

What Is Ozempic?
Ozempic is a prescription drug designed to control blood sugar levels for patients with type II diabetes, along with others such as Mounjaro, Wegovy, and Rybelsus. Developed in 2012 by Danish pharmaceutical company Novo Nordisk, Ozempic is classified as a GLP-1 receptor agonist analog-type drug. Ozempic contains the active ingredient semaglutide, a hormone inhibitor that stimulates the pancreas to release insulin to reduce blood sugar levels. It is distributed as a pre-filled pen to be injected underneath the skin once a week.
Ozempic Is Widely Prescribed as a Weight Loss Drug
In 2017, the U.S. Food and Drug Administration (FDA) approved Ozempic to treat adult diabetes. Despite its intended use, Ozempic is also widely prescribed as a weight loss drug for its ability to slow digestion and signal satiety and fullness to the brain, allowing patients to consume less and experience weight loss.
Side Effects of Ozempic
Ozempic, however, is causing many non-diabetic patients to develop severe illnesses, specifically gastroparesis (stomach paralysis). During delayed gastric emptying, food remains in the stomach for longer, which can cause stomach muscles to become paralyzed.
Patients experiencing gastroparesis feel full quickly and also suffer acute symptoms such as bloating, severe nausea, and vomiting. Stomach paralysis may also limit the body’s absorption of nutrients required from diet. The FDA has received numerous reports of gastroparesis from patients taking Ozempic and related drugs.
Ozempic is also linked to acute gallbladder disorders related to semaglutide. Since 2021, the FDA has received over 10,000 reports of severe adverse effects of the inhibitor.
Novo Nordisk rejects accusations of the drug’s safety, prompting multiple lawsuits claiming the pharmaceutical manufacturer failed to adequately warn doctors and patients of the potentially dangerous side effects of Ozempic.
